Disease
Modeling
In Platform 3, experimental and computational disease modeling will serve preclinical safety and efficacy studies for targeted therapies. We will employ:
– Computer models for optogenetic encoding to restore functions.
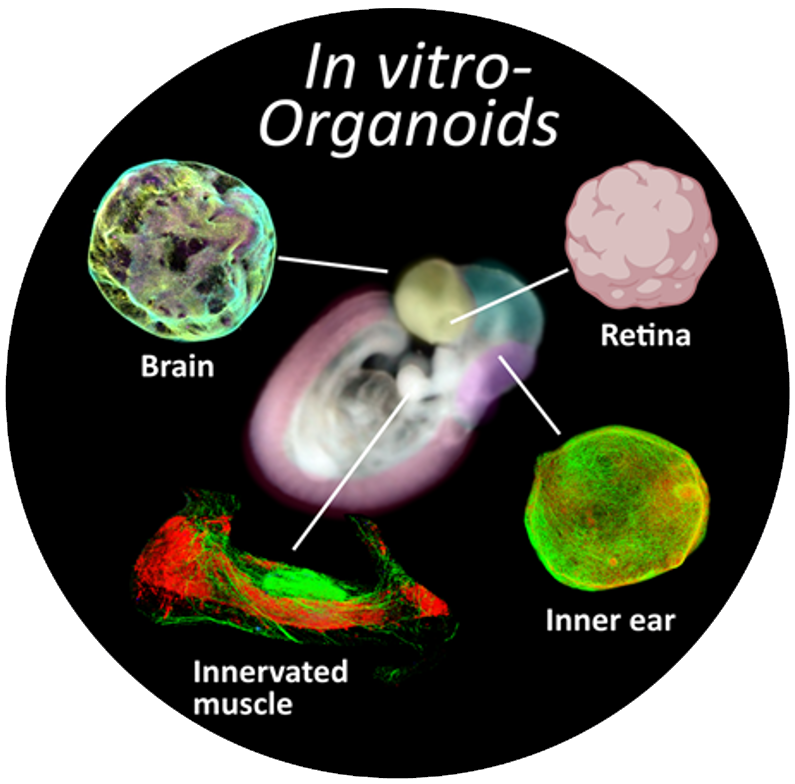
– Human neural organoids as preclinical disease models and screening platforms for efficient and cell-specific gene transfer in patients.
– In vivo models for various rodent and large animal species to test the efficacy, safety, and reliability of optogenetic therapies.
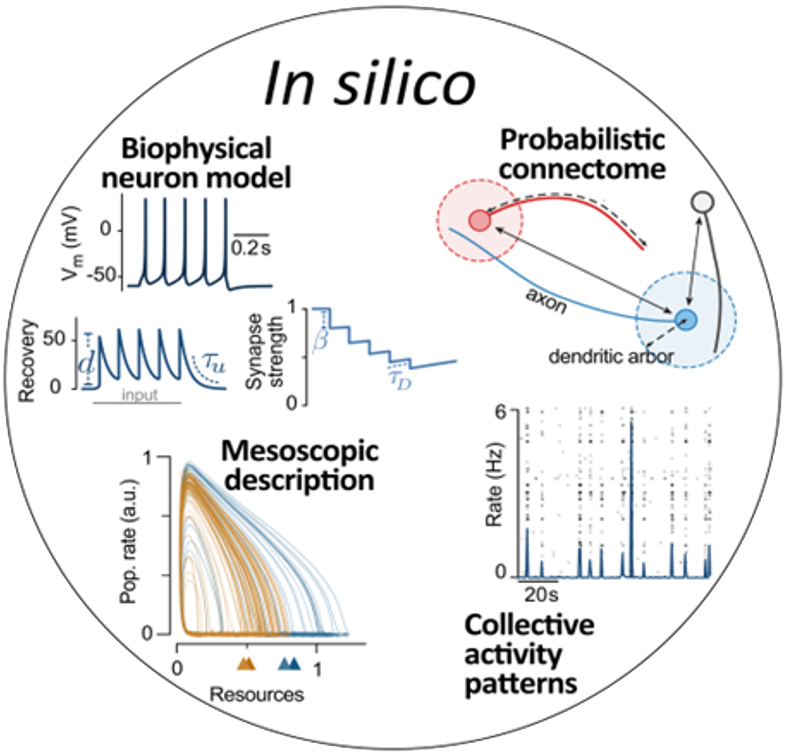
The in silico platform will use simulations and theories to understand how plasticity mechanisms interact with intrinsic and light-controlled stimuli to alter information processing in the targeted networks. This will involve biophysically interpretable models of neurons and synapses. Their parameters will be constrained by measurements from organoids and tissue slices. Simulations of activity in the networks lead to measurements of information processing for intrinsic and light-controlled input, which are then compared with multi-electrode array measurements in vitro and in vivo recordings. In silico, we can rapidly test a wider range of conditioning paradigms than would be possible in vivo. This leads to a better understanding at the mesoscopic level, the development of promising stimulation paradigms for optimized information encoding, and helps to reduce and refine animal experiments. Promising stimulation patterns will then be tested in the in vitro and in vivo platforms.
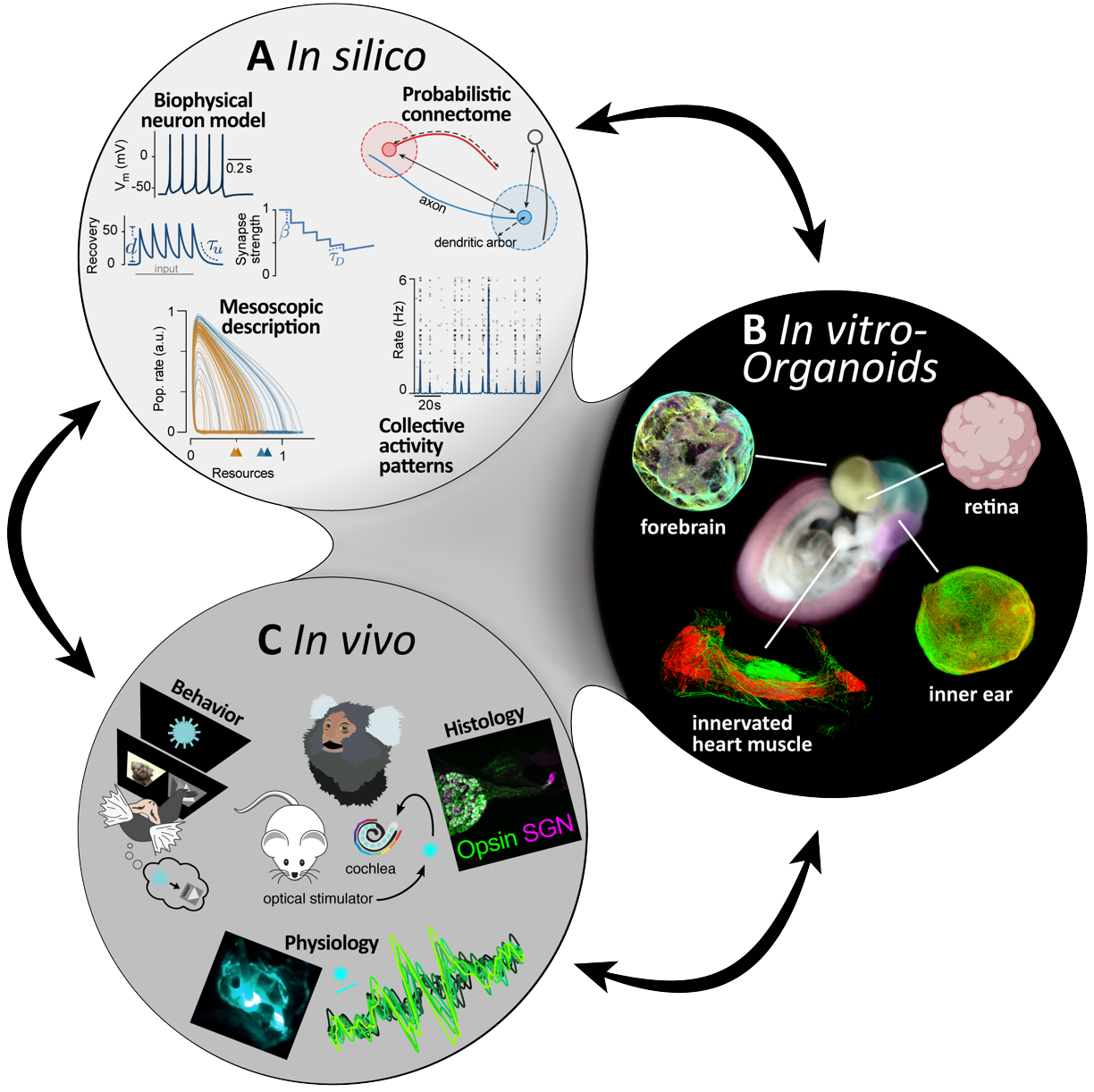
The in vitro platform will use established human iPSC-derived organoids and planned retinal organoids to identify optimal viral vectors/promoters for targeted cell stimulation through optogenetic methods. The efficacy of viral vector transduction will be validated through immunofluorescence, patch-clamp, and multi-electrode array recordings during optogenetic stimulations. The multi-electrode array data will be made available to the in silico platform to simulate network organization with and without optogenetic treatment. Promising combinations of viral vectors and promoters identified in human organoid screening will be further tested in the in vivo platform, reducing the number of experimental animals needed. The integration of data from the in silico and in vivo platforms will enable the optimization of optogenetic stimulations for in vitro experiments.
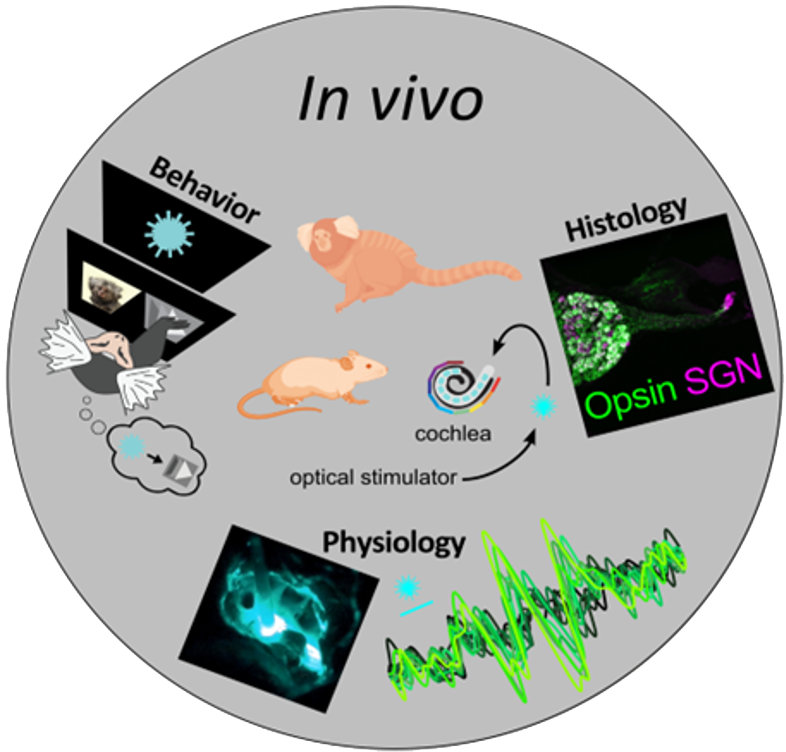
The in vivo platform will test the most suitable optogenetic therapy candidates in various model species such as rodents, pigs, and NHPs. The workflow will be illustrated using optogenetic hearing restoration, where a virus is applied to the cochlea and subsequently optically stimulated. Histological evaluation will determine optogene expression.