Team I
Optogenetic Hearing Restoration
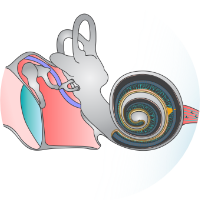
Hearing impairment is considered the most frequent human sensory deficit. More than 5% of the world’s population – 432 million adults and 34 million children – suffer from disabling hearing impairment. In severe cases, cochlear implants (CIs) partially restore hearing and provide open speech perception to most of the currently approx. 1 Mio. CI users. However, while electrical cochlear implants (eCIs) enable understanding of speech in a one-to-one conversation in the quiet in most users, communication in the presence of background noise largely fails. Moreover, eCI users typically do not have access to melodies in speech and music. Hence, there is a major unmet clinical need in 30 million profoundly hearing impaired and deaf people. This is largely attributed to the wide spread of electrical current from each of the 12-24 electrode contacts in the cochlea. Optogenetic stimulation overcomes this limitation: As light can be better confined in space, optical (o)CIs have greater frequency selectivity. This promises another major step to normality for these patients: communication in daily situations and participation in a normal social life. As a one-suits-all approach, oCIs will be more broadly available than future gene augmentation therapy for specific forms of genetic deafness.
We preclinically proved feasibility, stability, and efficacy of optogenetic stimulation of spiral ganglion neurons (SGNs): We generated fast-gating, red-light activated and found near physiological spectral selectivity and temporal fidelity in rodents using various AAV capsids as viral vectors. We performed preliminary tests of stability and safety of optogene therapy in mice with favorable results, which we are following up in gerbils and NHP. We now plan preclinical dose finding studies in gerbils and marmosets using microcatheter-based administration. Together with the L2T platforms, we will continue to develop, apply, and characterize advanced optogenes, AAVs, and promoters to prepare the next generation of optogenetic hearing restoration with emphasis on improved efficacy and the results of the 1st clinical dose escalation trial, planned from 2027 on. We expect a multicentric clinical trial of the new oCI to start in 2031 with the potential to transform hearing restoration for several decades to come.