Platform 3
Disease Modeling
In platform 3 experimental and computational disease modeling will serve preclinical safety and efficacy studies across the targeted therapies. We will employ:
• Computational models for optogenetic coding for functional restoration.
• Human neural organoids as pre-clinical models of disease and screening platforms for efficient and cell specific gene transfer in patients.
• In vivo model provision of various rodent and large animal species to test efficacy, safety, and reliability of optogenetic therapies.
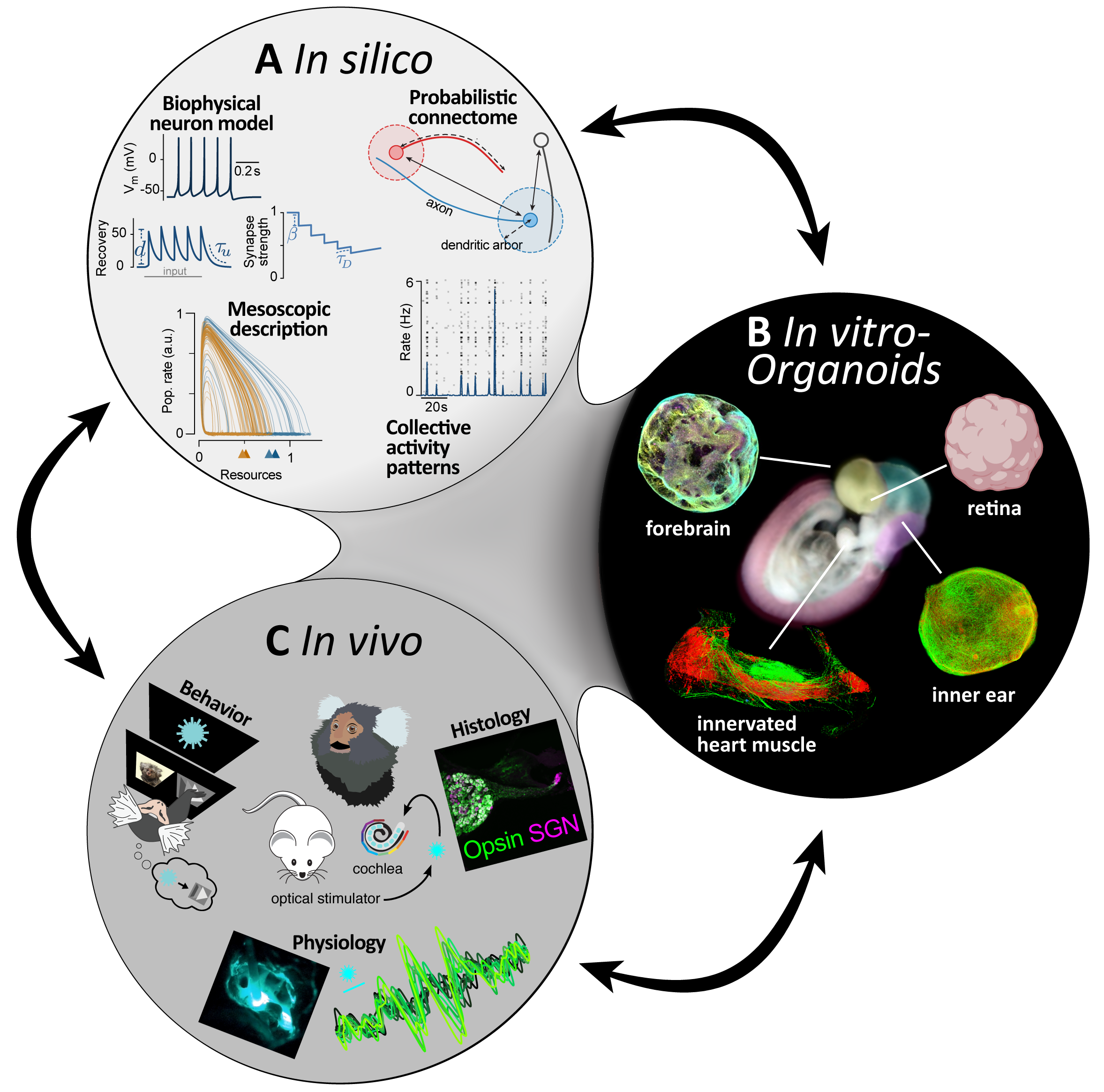
The in silico platform will use simulation and theory to understand how plasticity mechanisms interact with intrinsic and light-driven stimuli to change the information processing in the targeted networks. This uses biophysically interpretable models of neurons and synapses. Their parameters are constrained by measurements on organoids and tissue slices. Simulations of the activity in the networks leads to measures of information processing for intrinsic and light-driven input, again to be compared against multielectrode array measurements from in vitro and in vivo recordings. In silico, we can rapidly test a larger range of conditioning paradigms than would be possible in vivo. That leads to higher level, mesoscopic scale understanding, the development of promising stimulation paradigms for optimized information encoding and helps to reduce and refine animal experiments. Promising stimulation patterns will then be tested in the in vitro and in vivo platforms.
The in vitro platform will utilize established human iPSC-derived organoids and planned retinal organoids to identify optimal viral vectors/promoters for targeting cells for optogenetic stimulation. Efficacy of viral vector transduction will be validated by immunofluorescence, patch clamp and multielectrode array recordings upon optogenetic stimulations. Multielectrode array data will be provided to the in silico platform in order to simulate network organization with and without optogenetic treatment. Promising viral vector/promoter combinations identified by the human organoid screening will be further tested in the in vivo platform, thus reducing the number of experimental animals required. Integration of data from the in silico and in vivo platform will allow optimization of optogenetic stimulations for the in vitro experiments.
The in vivo platform will test most suitable optogenetic therapy candidates in various model species such as rodents, pigs and NHP. Workflow is illustrated with optogenetic hearing restoration involving viral application into the cochlea and subsequent optical stimulation. Histological evaluation will determine optogene expression.